Hundreds of drugs are being studied around the world, but “I don’t see a lot of home runs right now,” said Dr Carlos del Rio, a professor of infectious diseases at the Emory University Rollins School of Public Health. “I see a lot of strikeouts.”
Researchers have launched more than 1,250 studies of Covid-19. Pharmaceutical companies are investing billions to develop effective drugs and vaccines to help end the pandemic.
Dr Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, was cautious when announcing the results of a clinical trial of remdesivir earlier this month, noting it isn’t a “knockout.” Although remdesivir helped hospitalised Covid-19 patients recover more quickly, it hasn’t been proved to save lives.
“This (drug) is opening the door,” Fauci said. “As more companies and investors get involved, it’s going to get better and better.”
Researchers have already announced that they will combine remdesivir with an anti-inflammatory drug, baricitinib — now used to treat rheumatoid arthritis — in the hope of improving results.
But the new coronavirus is an elusive enemy.
Doctors treating Covid-19 patients say they’re fighting a war on multiple fronts, battling a virus that batters organs throughout the body, causes killer blood clots and prompts an immune system overreaction called a “cytokine storm.”
With so many parts of the body under siege at once, scientists say, improving survival rates will require multiple routes of attack — and more than one drug. While some of the experimental medications target the virus, others aim to prevent the immune system from inflicting collateral damage.
“There are so many pieces of this, and they will all require different therapies,” said Dr Lewis Kaplan, president of the Society of Critical Care Medicine, whose doctors provide intensive care.
High-tech approaches include using stem cells, virus-specific T cells and synthetic antibodies to neutralise the coronavirus.
Scientists are also taking a fresh look at existing medications that might be repurposed to fight Covid-19. These include antivirals for influenza, arthritis drugs, oestrogen patches and even antacids. If repurposed drugs are successful, they could reach patients relatively quickly, because doctors are already familiar with their side effects and safety concerns.
Some doctors are sceptical that drugs for heartburn or hot flashes have any chance of treating a killer like Covid-19.
Dr Steven Nissen, chair of cardiovascular medicine at the Cleveland Clinic, said he fears that hype over unproven products will harm patients, even if it temporarily boosts company stock prices. Patients who demand antacids or antimalarial drugs being studied in Covid-19 could be harmed by side effects, for example. Those who hoard drugs — on the hope of protecting themselves from Covid-19 — could deprive other patients of medications they need to stay healthy. Some people may refuse to participate in clinical trials because they fear being given a placebo.
“This rush to get every imaginable treatment into a study, it’s not prudent,” Nissen said. “It’s not good medicine. It’s an act of desperation.”
Other experts say scientists should cast a wide net.
“I don’t think we want to rule anything out because it sounds out of the ordinary,” said Dr Walid Gellad, director of the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh.
Antivirals such as remdesivir aim to prevent viruses from replicating, said Dr Peter Hotez, a professor at Baylor College of Medicine in Houston.
That doesn’t always work. A small Chinese study of remdesivir, published last month in The Lancet, found no benefit to severely ill Covid-19 patients. Remdesivir had previously failed when tested against Ebola.
Antivirals tend to be most helpful in the early stages of infection, when most of the harm to the patient is caused by the virus itself, rather than the immune system, Hotez said.
Remdesivir is just one of many antivirals being tested against Covid-19.
International researchers are studying the antiviral favipiravir, developed to fight the flu.
The antimalarial drugs chloroquine and hydroxychloroquine — which have been heavily touted by President Donald Trump — also have antiviral effects. Although the Food and Drug Administration approved forms of those drugs for emergency use against Covid-19, the agency later warned that they could cause dangerous heart rhythm problems.
A study in the New England Journal of Medicine likewise found no benefit in giving two antivirals used to treat HIV — a combination of lopinavir and ritonavir, sold as Kaletra — in adults hospitalised with severe Covid-19.
One of the therapies generating excitement is also one of the oldest: antibody-rich blood from Covid survivors.
The immune system produces antibodies in response to invaders such as viruses and bacteria, allowing the body to recognise and neutralise them. Antibodies also recognise and neutralise the virus the next time that person is exposed.
Doctors hope that patients who develop antibodies against the novel coronavirus will become immune, at least for a few years, although this hasn’t been proved.
Scientists developing this “convalescent plasma” are studying whether Covid-19 survivors can share this immunity with others by donating their plasma, the liquid part of blood that contains antibodies, said Dr Shmuel Shoham, an associate professor of medicine at the Johns Hopkins University School of Medicine.
In addition to treating people who are already sick, donated plasma could potentially prevent people exposed to the virus — such as health care workers — from developing symptoms.
Donated antibodies — and any immunity they might provide — don’t last forever, said Dr William Schaffner, a professor at the Vanderbilt University Medical Center. The body destroys ageing antibodies as part of its routine maintenance, he said. In general, half of donated antibodies are eliminated in about three weeks.
The use of convalescent plasma goes back more than a century. It was used during the 1918 flu pandemic and was shown to improve survival during the 2009-10 H1N1 pandemic.
Doctors don’t know yet whether convalescent plasma will benefit people with Covid-19.
In general, convalescent plasma is expected to be more effective in preventing illness than in treating it. It may be less likely to help someone in intensive care, Shoham said.
Researchers are also studying the use of prepackaged plasma, called intravenous immunoglobulin, in Covid patients. This product, known as IVIG, is taken from healthy donors in the general population and has long been used to help patients with weakened immune systems fight off infections. Hospitals keep it in stock and some are already using it to treat Covid patients.
Although the antibodies in prepackaged IVIG don’t specifically target the coronavirus, researchers hope they will tamp down the immune response.
In a third form of immune therapy, researchers are trying to identify the specific antibodies that are most important for neutralising the coronavirus, then reproduce them as drugs called monoclonal antibodies. Monoclonal antibodies are already used to treat a variety of conditions, from cancer to rheumatoid arthritis and migraines.
“When we give people an antibody, they are immediately at least partially immune to that specific virus,” said Dr James Crowe, director of the Vanderbilt Vaccine Center, who hopes to have antibodies ready for a clinical trial in a few months. “We’re moving the immune system from one person to another.”
Ideally, doctors would develop a very potent monoclonal antibody or a cocktail of antibodies for Covid-19 patients, to ensure the best chance of success, Crowe said. But manufacturing these drugs can be complicated, expensive and time-consuming.
“Making two antibodies would be at least twice as complicated as making one,” Crowe said. “A cocktail might be preferred, but cocktails are harder to move quickly.”
In most cases of Covid-19, the immune system neutralises the coronavirus and patients recover without going to the hospital.
For reasons that doctors don’t totally understand, the immune system of some Covid-19 patients becomes hyperactive, attacking not just the virus but the patient’s own cells. A “cytokine storm,” in which the immune system floods the body with inflammatory chemicals, can do more damage than the virus itself.
In an effort to calm the immune system, researchers are testing immune-suppressing drugs, including monoclonal antibodies already used to treat autoimmune diseases such as rheumatoid arthritis, said Dr Amesh Adalja, a senior scholar at the Johns Hopkins Center for Health Security.
Healthcare giant Roche is conducting large clinical trials of its drug, Actemra, in the hope of preventing cytokine storms, which can cause organ failure and a life-threatening condition called sepsis. Actemra is designed to lower levels of an inflammatory chemical, interleukin-6, which has been found to be elevated in some Covid-19 patients.
Scientists are also studying similar drugs, anakinra and siltuximab.
Another immune suppressant from Regeneron and Sanofi, called Kevzara, has had disappointing results in clinical trials. The manufacturers plan to continue studying the drug to see if it can help certain types of patients.
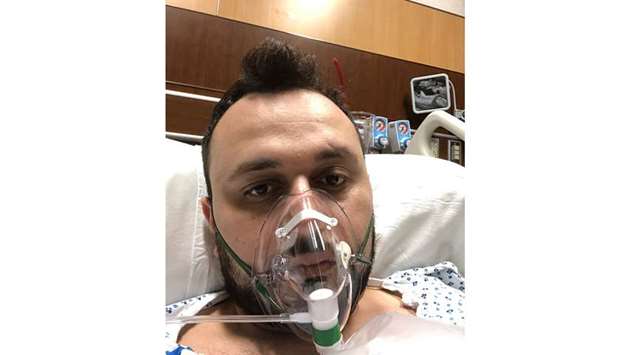
Dr Anar Yukhayev, a New York OB-GYN who was hospitalized with Covid-19 on March 16, agreed to join a clinical trial of Kevzara.
“I was having so much trouble breathing that I was desperate for anything to help,” said Yukhayev, 31, who was treated at Long Island Jewish Medical Center.
About 36 hours after receiving an infusion, as Yukhayev was being treated in intensive care, his symptoms began to improve. He was able to avoid being put on a ventilator. Doctors didn’t tell him if he received Kevzara or a placebo, but his liver enzymes also began to rise, suggesting the organ was under stress. Elevated liver enzymes are a known side effect of Kevzara.
Yukhayev made a full recovery and went back to work full time April 13. He donated his plasma to researchers.
Until vaccines and other preventive medicines are developed, the best way to prevent coronavirus infections is to maintain social distancing, Adalja said.
“Social distancing is a blunt tool,” he said, “but it’s all that we have.”
— Kaiser Health News/TNS