If ears were burning across the medical technology industry last month, it might have been Jeanne Lenzer’s fault.
Lenzer, an investigative journalist and longtime contributor to the BMJ medical journal, has a new book out called The Danger Within Us: America’s Untested, Unregulated Medical Device Industry and One Man’s Battle to Survive It (Little, Brown; 336 pages), which lays bare a system that allows most medical devices to go onto the US market without rigorous testing.
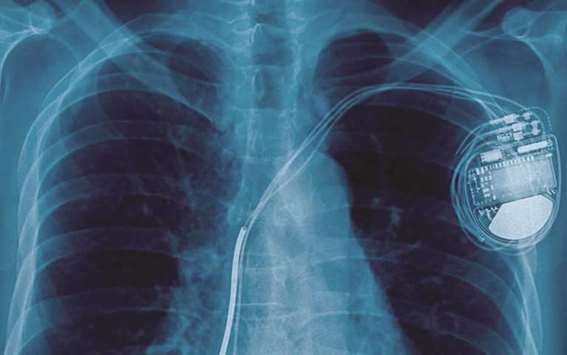
Even when randomised controlled trials are done, statistics can be manipulated and negative results can be suppressed. The book (which briefly cites investigative reports in the Star Tribune) tells the story of Dennis Fegan, who doctors said nearly died from problems caused by a nerve stimulator implanted in an effort to regulate seizures. Following is an edited transcript of an interview with Lenzer.
Your book is pretty tough on med-tech companies. How has the reaction been from the med-tech community?
They say the book is crap, according to a couple of people who responded on Amazon! (laughs) But it’s been overwhelmingly positive from both the lay and physician community. A number of people are simply shocked that the (Food and Drug Administration) doesn’t do more by way of vetting these devices. That came as a real surprise, not only to patients, but to many physicians.
So why do medical device companies talk about the opposite phenomenon? They say it can be very difficult to get products through the FDA.
I think the distinction is, when I say they don’t do clinical trial testing, they read that as they never did any testing at all. … Yes, they fill out binders of information. But what they generally don’t have — almost never have — is clinical trial data.
Why is that?
They feel that they can rely on biological plausibility: If you have a clogged coronary artery and you open it with a stent, that must be a good thing. Except that we’re learning it isn’t, necessarily, particularly in the case of stable angina. And it’s not just clinical trial testing. We need mandatory registries (of implanted devices), which we don’t have in this country, but that do exist in some other countries. So what you can’t catch in clinical trials then at least we should be catching with registries.
Do you think the issue could be resolved by better informing the public about how many people have had the same device before they did, and how much testing has gone into it?
I think that’s very difficult. Device companies have been able to fool doctors, so I’m not sure that patients going to fare any better. I think the answer is that we need independently conducted clinical trials or at least independent analyses of these trials, rather than relying on the companies to tell us what works and doesn’t work.
When a patient has positive health outcomes, those outcomes tend to get attributed to the device being tested. But when people get hurt, often the doctor or the underlying disease are blamed. Why does that happen?
One of the things I learned in writing this book is how often the cure really is the cause of many types of adverse events. It’s hard to distinguish. If a patient is having more seizures, how do we know it’s not just his condition getting worse, vs. an adverse effect of the device? It’s naturally occurring because every drug and device targets the very body systems and physiological systems that are already disordered. In Dennis Fegan’s case, (the manufacturer, Cyberonics, was) confronted with the fact that three doctors all saw the same thing, that his heart stopped exactly at 3-minute intervals, which is precisely when his device was set to fire — at 3-minute intervals — and that all his seizures stopped immediately when the device was turned off, and didn’t return. Despite that, the company tried to blame a medicine that he was on, rather than their device. The problem is that device companies are allowed to determine whether their device is related to the cause of death or not, or a serious adverse event.
Are you concerned that doing more rigorously controlled studies might make some devices more expensive, or impossible to get to the market?
Sure it will make them more expensive, and sure it will make some of them more difficult to get to market — thank goodness, for the ones that should be kept off the market. And as for more expensive? What it will do is save the billions of dollars that we are now spending on devices that don’t work and are dangerous, and the subsequent lawsuits. I had a surgeon who told me, you know what? Device manufacturers could cut a piece of curtain and hand it to us and tell us its surgical mesh, and we won’t know the difference. I mean, sometimes surgeons are the victims in this, too. They don’t know. We want to know the difference between devices that are truly life-altering, beneficial, lifesaving in some instances — and those that are dangerous. And the only way we’re going to know is by having a more rigorous scientific approach to approving devices and monitoring them. And it’s a stinking shame we don’t.— Star Trubune
.
