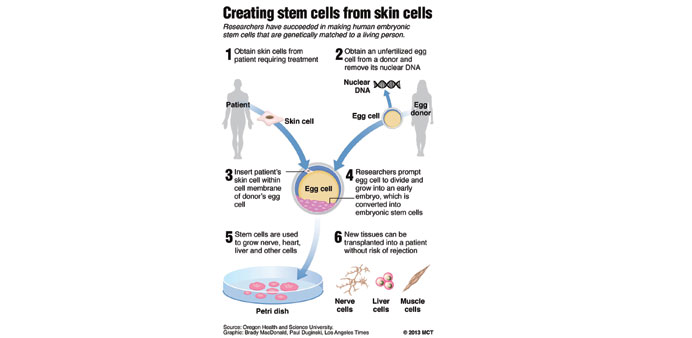
For first time, stem cells are produced from cloning technique. By Melissa Healy
|
|
For the first time, scientists have created human embryos that are genetic copies of living people and used them to make stem cells — a feat that paves the way for treating a range of diseases with personalised body tissues but also ignites fears of human cloning.
If replicated in other labs, the methods detailed in the journal Cell would allow researchers to fashion human embryonic stem cells that are custom-made for patients with Alzheimer’s disease, diabetes and other health problems.
Theoretically capable of reproducing themselves indefinitely, these stem cells could be used to grow replacements for a wide variety of diseased cells — those of the blood, skin, heart, brain, muscles, nerves and more — that would not risk rejection by the patient’s immune system.
The report also raises the spectre that, with a high-quality donor egg, a bit of skin, some careful tending in a lab and the womb of a willing surrogate, humans have cracked the biological secret to reproducing themselves. That is an objective American scientists have squarely renounced as unethical and scientifically irresponsible. At the same time, most acknowledge that such “reproductive cloning” will one day prove too tempting to resist.
In the hope that other researchers will validate and extend their results, the scientists at Oregon Health & Science University (OHSU) provided an exceptionally detailed account of their techniques. But for anyone with a well-equipped fertility lab, the comprehensive guide could be a useful handbook for cloning a baby.
OHSU cell biologist Shoukhrat Mitalipov led a team of 23 scientists who methodically culled the lessons learned from stem cell research on amphibians, mice and rhesus monkeys — as well as from the abundant failures of others in the field. They devised a welter of new techniques to use the DNA of a fully formed skin cell in its most primitive embryonic form.
The approach they used — called somatic cell nuclear transfer — effectively strips an egg of its chromosomes and packs it instead with DNA from a donor.
Nurtured by a stew of nourishing chemicals and zapped with two jolts of electrical current, many of the eggs began to divide and grew for five to six days. At that point, the embryos had 64 to 200 cells, including a dense inner cell mass from which stem cells were extracted.
In past efforts to coax such an assemblage of components to life, researchers have burned through dozens of donor eggs without getting any embryos even to the 16-cell stage at which stem cells become a remote possibility.
This time, the researchers said their methods were so efficient that they could create at least one embryonic stem cell line from each batch of eggs donated by 10 female volunteers. In one case, a single donor produced eight eggs of such exceptional quality that researchers were able to derive four embryonic stem cell lines.
The volunteers, between the ages of 23 and 31, donated their eggs anonymously and were “financially compensated for the time, effort, discomfort and inconvenience associated with the donation process,” the study authors wrote.
The success of the experiments rekindled debate among bioethicists, who have long anticipated that human cloning would become a reality. In 2002, a commission of bioethicists established by then-President George W Bush unanimously urged a ban on reproductive cloning. But the panel was deeply divided about the propriety of “therapeutic cloning” for research and medical treatment.
Though 13 states have passed laws banning reproductive cloning, the United States is one of just a few industrialised countries that has not prohibited the practice. Seven states also have banned therapeutic cloning. Oregon is not one of them.
The OHSU team’s success underscores the urgent need for federal rules that spell out consistent national limits on therapeutic cloning and put a clear ban on the technology’s use in fertility clinics, said Johns Hopkins University bioethicist Jeffrey Kahn.
Researchers are also likely to step up their demand for donated eggs so they can conduct similar experiments. That lends urgency to the need for standardised practices for compensating women who donate their eggs. Some states have set strict limits on such payments, while others have allowed a market for donated eggs to flourish unregulated, Kahn added.
Among the methodological innovations outlined in the Cell paper was a trick that stem cell scientist Michael D West, who was not involved in the study, dubbed “the Starbucks effect”. The OHSU team added caffeine to the growth medium that nourished the eggs after they were stripped of their original DNA and awaited the new DNA from a skin cell. Unlike its stimulating effect on coffee-drinkers, the caffeine chemically slowed the rush to divide and grow that had doomed earlier efforts.
The OHSU scientists also studied which batches of donated eggs were most likely to thrive and survive long enough to produce stem cells. Finding that eggs fared best when they were part of a medium-sized harvest, the researchers fine-tuned their regimen of egg-stimulating drugs so that more of the women produced roughly 10 eggs per cycle.
In all, the researchers produced six lines of embryonic stem cells from six separate embryos, said OHSU infertility specialist Dr Paula Amato, one of the paper’s authors. Four of them contained the nuclear DNA of a single donor whose skin cells were purchased from a commercial lab, and a fifth came from a separate donor whose cells were bought the same way. The sixth donor was a patient with Leigh syndrome, a genetic disorder that results in severe and mostly fatal neurological degeneration in a child’s first years of life.
The embryonic stem cell lines faithfully reproduced the nuclear DNA of the person who contributed the skin cell. The expectation is that when cells or tissues made from these stem cells are transplanted into that person, they will slip past his or her immune system unnoticed.
The stem cells showed “no gross chromosomal abnormalities,” the authors wrote, and appeared every bit as capable of differentiating into a variety of cell types as embryonic stem cells made the old-fashioned way, from excess in vitro fertilised embryos obtained from fertility clinics.
“This is extremely important,” said Dr Irving Weissman, a stem cell researcher at Stanford University.
Stem cell researcher Dr Robert Lanza echoed that praise, calling the OHSU team’s work “a major scientific achievement.”
Lanza, the chief scientific officer of Advanced Cell Technology Inc, said it remained to be seen whether embryonic stem cells generated this way were more useful for studying and treating diseases than stem cells created by reprogramming adult cells to an embryonic state.
The method for creating such induced pluripotent stem cells, or iPS cells, was recognised with a Nobel Prize last year.
But there are lingering questions about whether iPS cells are safe for patients. Scientists use a cancer-causing virus to reprogram the cells, raising concerns that they could cause tumours in patients. And in 2011, when scientists at the University of California, San Diego, created iPS cells and reintroduced them into donor mice, the animals’ immune systems rejected them.
“Some experts are now saying that somatic cell nuclear transfer may be the only way to truly reprogram cells,” Lanza said. — Los Angeles Times/MCT