The US Food and Drug Administration (FDA) approved on Monday Abrysvo (Respiratory Syncytial Virus Vaccine), the first vaccine approved for use in pregnant individuals to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by respiratory syncytial virus (RSV) in infants from birth through 6 months of age.
Abrysvo is approved for use at 32 through 36 weeks gestational age of pregnancy. Abrysvo is administered as a single dose injection into the muscle.
The FDA approved Abrysvo in May for the prevention of LRTD caused by RSV in individuals 60 years of age and older.
Globally, RSV causes 160,000 deaths each year. Infants are at the greatest risk of severe RSV.
The CDC estimates RSV causes 58,000 to 80,000 annual hospitalizations of children younger than 5 years old.
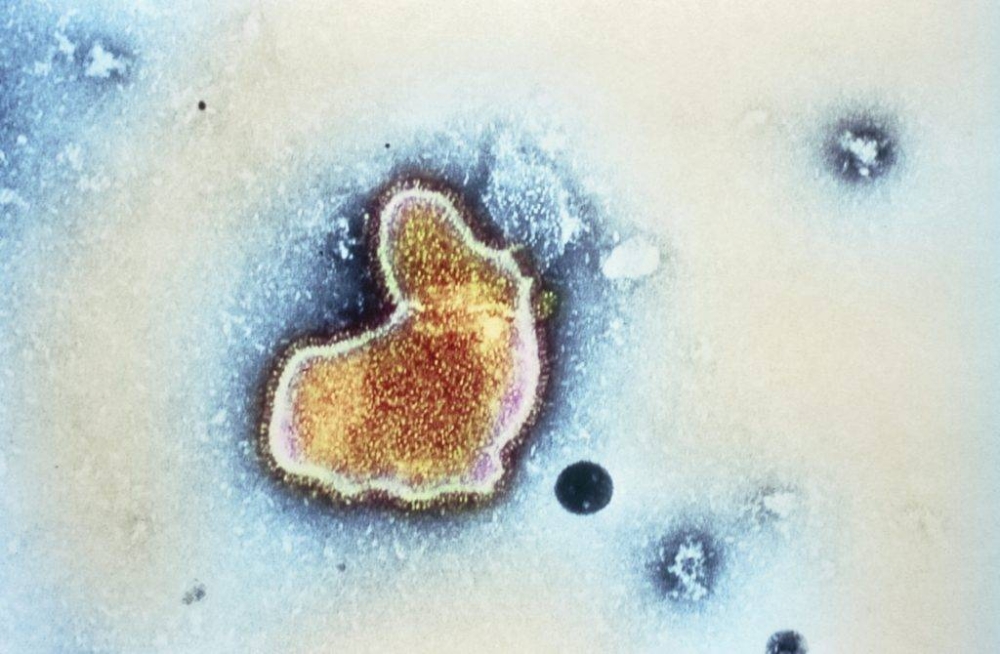
Respiratory syncytial virus. Coloured transmission electron micrograph (TEM) of a respiratory syncytial virus (RSV). This pneumovirus, a type of paramyxovirus, is a major cause of human respirat- ory tract infections in temperate climates, especially in winter. The virus consists of RNA (ribonucleic acid) genetic material enclosed in a protein coat, or capsid, within a phospholipid envelope. The envelope is covered in protein spikes, seen as the yellow lines around the edge of the virus. In adults, the virus only affects the upper respiratory tract, but in infants bronchiolitis (bronchiole inflammation) or bron- chopneumonia can result. Magnification unknown.
