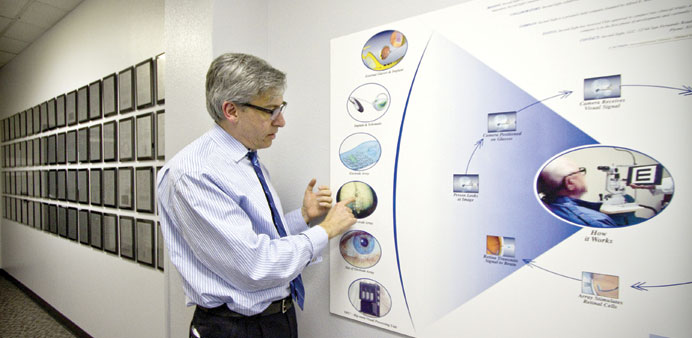
Called the Argus II Retinal Prosthesis System, the unit includes a small video
camera, a portable computer and a tiny implant that is placed near the retina
during a two-hour surgical procedure. By Ronald D White
|
|
Robert Greenberg got tired of hearing from senior engineers that it wasn’t possible to build his product idea: a bionic eye that gives sight to the blind.
“A lot of the folks straight out of school didn’t know any better, so I hired them instead,” quipped Greenberg, chief executive of Second Sight Medical Products Inc, a Sylmar, California, biotech company. “They didn’t know how hard it was going to be, that it was impossible. And so they tried.”
Greenberg can laugh now that he once thought developing the device would take a year and $1mn. Some 20 years and $200mn later, the first bionic eye has helped more than 20 European patients regain some of their sight.
Called the Argus II Retinal Prosthesis System, the device recently was approved by the US Food and Drug Administration. Second Sight, which has 100 employees, is allowed to sell the bionic eye system to patients in the US with advanced retinitis pigmentosa, a degenerative eye disease that can cause blindness.
“We are a far cry from restoring 20/20 vision,” said Brian V Mech, Second Sight’s vice president of business development, who holds a doctorate in materials science and an MBA from the UCLA Anderson School of Management. “We are taking blind people back up to low vision, and that is pretty significant.”
Mech likes to show videos of once-sightless patients who, after receiving the retinal prosthesis, are able to follow a person walking down the street and discern a street curb without using their canes. “Until our product, these patients had no other option to obtain the ability to see,” Mech said of the $100,000 device, part of which rests on a pair of Oakley Inc sunglass frames. The cost to European patients has been paid by insurance companies in most cases.
Palo Alto attorney Dean Lloyd, who lost his vision 17 years ago, got the bionic eye system as part of the US testing process. It allows him to see “boundaries and borders, not images” but has had a profound effect on his life.
Lloyd cites an incident before he received the eye system that still rankles. In the middle of a courtroom trial, an opposing attorney said Lloyd didn’t stand a chance with his case because he couldn’t even keep his socks straight: Lloyd had mixed up his black, courtroom socks with his white athletic ones.
“What did I do after the surgical procedure that I hadn’t been able to do?” Lloyd said. “I went home and sorted all of my socks.”
The story of how the bionic eye came to be made in Sylmar underscores the state’s long record of medical device advances and involves top university researchers who were brought to Southern California to work on the project.
Greenberg likened the degree of difficulty to “shrinking a television set to the size of a pea, then throwing it into the ocean and expecting it to work.”
For Greenberg, it began in the early 1990s when he was a doctoral candidate in the Department of Biomedical Engineering at Johns Hopkins University in Baltimore.
Some of the first work was being done there, testing patients who had lost their vision because of retinitis pigmentosa, to see if electrically stimulating their retinas would produce results. It did.
“Using one electrode, the patient saw one spot of light,” Greenberg said. “Second electrode, and the patient was seeing two spots of light. During that experiment, I was hooked.”
Greenberg said he thought: “This is just engineering. Put more spots and you could make more pixels, like lights on a scoreboard or pixels on your computer monitor. You could see images.”
There was a breakthrough of another sort a few years later, in Washington. There, Greenberg was working as a medical officer and a lead reviewer for the FDA’s Office of Device Evaluation when he met entrepreneur Alfred E Mann.
Mann had already established himself as a medical device developer through Mannkind Corp and several other Southern California companies. During the 1980s, the self-made billionaire founded Pacesetter Systems, which made cardiac pacemakers. From there, he moved on to insulin pumps and related equipment.
Another Mann-funded company, Advanced Bionics Corp, took on cochlear implants, which could restore hearing to the deaf. It was the electrode-based cochlear implant that formed the rough basis of Second Sight’s first bionic eye.
In 1998, Second Sight opened with the financial backing of Mann and Sam Williams, another successful entrepreneur whose company, Williams International, designed and built small, efficient turbofan jet engines.
“Sam Williams was blind from retinitis pigmentosa, the disease that we are treating,” Mech said. “He had invested along with Al in Advanced Bionics, which restores hearing for deaf people, and they were already on the market in the '90s. Sam said to Al, ‘Why can’t we do the same for blind people?’”
Mann and Williams weren’t the only ones who recognised that the technology behind the bionic eye held promise. In 2001, the University of Southern California recruited the two doctors that Greenberg had worked with at Johns Hopkins.
There was more to come. Greenberg was still flying back and forth to Baltimore and Johns Hopkins when Steven J Ryan, president of USC’s Doheny Eye Institute, put up the money to “move our entire Johns Hopkins team out here to Los Angeles,” Greenberg said.
“It was a big deal, five people and their families,” Greenberg said. “The work would not have progressed as fast if that hadn’t happened. It was part of how we got a dedicated team focused on this one project.”
The Argus II has three parts. One part includes a small video camera that sits on sunglasses. Another part is a portable computer, which can be worn on a belt or carried in a purse.
The most complicated part is a tiny implant that is placed near the retina during a two-hour surgical procedure. The implant carries 60 electrodes, up from 16 in the first version.
The computer processes signals from the video camera and wirelessly relays the information back to the implants.
The bionic eye system requires an unusual manufacturing process involving highly trained engineers working with microscopes to manually connect each of the 60 electrode arrays.
The microscopes range from relatively low power models, normally used for dissections, to high-powered metallurgical scopes, all the way up to scanning needles that can measure at the nanometer level. “Almost every step is done under a microscope,” Greenberg said. The lines on the retinal implants are only 25 microns, or 1/1,000th of an inch wide.
There are additional manufacturing problems when the product you are making involves such small scales. Regular platinum will not work in such small electrodes and would actually dissolve, Greenberg said. Second Sight had to develop a different platinum-based material.
All of the manufacturing work is also done in clinical-standard clean rooms, with each of the engineers so fully suited up that they look like biohazard technicians from the Centers for Disease Control and Prevention. A single speck of dust could ruin the device.
Part of the work is done under yellow lights, which act as a kind of photography darkroom during a part of the process that involves a photographic plate, Greenberg said. Much of the process is patented and proprietary. In fact, Second Sight already has more than 200 patents in the US and Europe, and more than 100 others are pending.
And when you are building something for the first time that has to endure the hot, salty environment of the eye, the means to test the device can’t just be found on a store shelf. The test devices too had to be manufactured by Second Sight. Some of the most important devices subject the implants to temperatures much higher than they will encounter in the eye and to movements that are much more severe than eye movements.
The venture has tapped into a storied history, Greenberg said, noting that some of the most important medical devices from Mann’s companies were made in the Sylmar facilities.
“The first rechargeable pacemakers were made here. The insulin pumps were also made right here, in these clean rooms,” Greenberg said. “This place has incredible karma.” — Los Angeles Times/MCT
Cover photograph: A technician in the clean room, under yellow light, inspects electrodes used on the Argus II Retinal Prosthesis at Second Sight Medical Products.